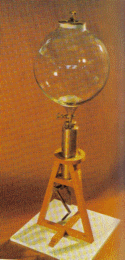SKC Films Library |
| SKC Films Library >> Science >> Physics >> Biography |
| Robert Boyle developer of the law which describes the relationship between the volume of a gas and its pressure
The 14th child of the Earl of Cork was born in the family castle in Lismore, Ireland, in 1627. As a child, he traveled widely in Europe and spent several months in Italy, where he was profoundly influenced by the writings of Galileo. In the 1640's, Boyle helped found an association of British scholars dedicated to furthering experimental science. Originally named The Invisible College, it received a Royal Charter in 1663 and was renamed the Royal Society. About 1659, Boyle became interested in the investigations then being carried out on the nature of vacuum. He designed and constructed a new kind of air pump, which he used to create a near vacuum so that he could study the phenomenon for himself. An apparatus could be placed in the globe-like receiver at the top, which had a stopper that could be made airtight by means of cement. When the globe was evacuated, flames were extinguished and any animal placed inside perished. reconstruction of the Boyle-Hooke
vacuum pump Using his apparatus, Boyle was able to prove Galileo's assertion that, in the absence of air resistance, bodies of different weights fall at the same rate. He also discovered that sound is dependent on air for its transmission, and that even the loudest noise is barely transmitted in a near vacuum. In another experiment, Boyle demonstrated the greatest height to which water could be raised by pumping. By standing on a roof 30 feet above a reservoir of water and sucking up the water with a pump, Boyle showed that because of atmospheric pressure there was a maximum height beyond which water could not be drawn up. demonstrating the greatest height
to which water can be pumped Boyle's experiments culminated in 1662 in the discovery of what is now known as Boyle's Law, which describes a simple but important inverse relationship between the volume of a gas and its pressure. Boyle found that if a certain quantity of gas was kept at constant temperature and the pressure was doubled, its volume was halved. If the pressure was increased three-fold, the volume was reduced to a third. Boyle's Law is still used today to calculate the way in which the pressure and volume of gases vary. Taking his discovery even further, Boyle concluded that since air could be compressed, it must be made up of tiny particles. Rejecting the belief that all matter consisted of combinations of the four elements -- earth, air, fire, and water -- he proposed instead that matter consisted of "primary particles" that could collect together to form "corpuscles." This idea of "primary particles" forming "corpuscles" anticpated the modern chemist's view of atoms forming bonds with each other to produce molecules. In 1680, Boyle was offered the presidency of the Royal Society but declined on obscure religious grounds. On his death, which came in 1691, he left money for a series of scholarly lectures dedicated to "proving the Christian Religion against notorious Infidels." These Boyle Lectures still continue today. SEE ALSO |
| SKC Films Library
>> Science >> Physics >> Biography This page was last updated on 10/24/2017. |