SKC Films Library SKC Films Library |
| SKC Films Library >> Science >> Chemistry >> Physical Chemistry |
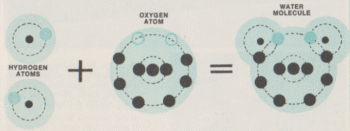
| How a Water Molecule is Formed A water molecule is formed when two atoms of hydrogen and one oxygen atom fill vacancies in their electron orbits by sharing electrons. Each hydrogen atom has only one electron orbiting its nucleus and needs one more electron to become stable. The oxygen atom has six electrons in its outer shell and needs two more to fill the orbit and achieve stability. When the three unstable atoms get together and "pool" their electrons, they form a stable molceule of water. Unfortunately for drought-prone areas, simply mixing hydrogen and oxygen together will not make water, as the orbits of each atom's electrons must become linked, and it takes a sudden burst of energy for that linking to occur. Since hydrogen is extremely flammable and oxygen readily supports combustion, it doesn't take much energy to create one water molecule; a single spark will suffice, it doesn't even have to be a flame. But by the time you have created enough energy to create a measurable amount of water you have created an explosion. WEB SOURCES |
| SKC Films Library
>> Science >> Chemistry >> Physical Chemistry This page was last updated on 05/18/2017. |