SKC Films Library |
| SKC Films Library >> Science >> Chemistry >> Biography |
| Henry Cavendish the first to recognize hydrogen as a distinct substance
Henry Cavendish was born at Nice, France, on October 10, 1731. He was sent to school at Hackney in 1742, and in 1749 entered Peterhouse, Cambridge, which he left in 1753 without taking a degree. A recluse throughout his life, Cavendish disliked the company of men and was terrified of women. His female servants were forbidden to cross his path and he communicated with them by handwritten notes. His sole pleasure in life was science, and he devoted himself to research with an enthusiasm bordering on obsession. He was a regular attendant at the meetings of the Royal Society, of which he became a fellow in 1760, and he dined every Thursday with the club. He also had a library where he would attend on appointed days to lend books to men who were properly vouched for. Otherwise, he had little intercourse with society. Cavendish's scientific work was wide in its range. The papers he himself published form an incomplete record of his researches, since many of the results he obtained became generally known only years after his death. Cavendish's first communication to the Royal Society was a chemical paper on "Factitious Airs" (Philosophical Transactions, 1766). This paper dealt mostly with "inflammable air" (hydrogen), which he was the first to recognize as a distinct substance, and "fixed air" (carbon dioxide). He determined the specific gravity of these gases with reference to common air, investigated the extent to which they are absorbed by various liquids, and noted that the air produced by fermentation and putrefaction has properties identical with those of fixed air obtained from marble. He introduced new refinements into his experiments, such as the use of drying agents and the correction of the volume of a gas for temperature and pressure. Cavendish's experiments on
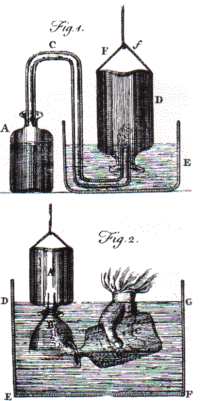
"factitious" air. Figure 1 demonstrates the
collection of the gas. Figure 2 shows the gas being
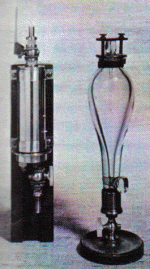
transferred to a storage vessel. In 1767 Cavendish published a paper on the analysis of one of the London pump waters, which showed that the calcareous matter in that water is held in solution by the "fixed air" present and can be precipitated by lime. In 1783 Cavendish described observations he had made to determine whether or not the atmosphere is constant in composition. After testing the air on nearly 60 different days in 1781, he could find, after 400 determinations in the proportion of oxygen, no difference of which he could be sure, nor could he detect any sensible variation at different places. Two papers on "Experiments on Air" (Philosophical Transactions, 1784 and 1785) contain his discoveries of the compound nature of water and composition of nitric acid. Starting from an experiment, narrated by Joseph Priestley, in which John Warltire fired a mixture of common air and hydrogen by electricity, with the result that there was a diminution of volume and a deposition of moisture, Cavendish burned about two parts of hydrogen with five of common air and noticed that the only liquid product was water. In another experiment he fired by electric spark a mixture of hydrogen and oxygen in a glass globe and again obtained water. Proceeding with these experiments he found that the resulting water contained nitric acid. In the second of the two papers he gives an account of the methods by which the composition of nitric acid was discovered. He observed also that a small fraction of the "phlogisticated air" of the atmosphere differed from the rest. In this residue he most likely had a sample of the inert gas argon, which was only recognized as a distinct entity more than 100 years later by J.W. Rayleigh and Sir William Ramsay. Cavendish's spark eudiometers for
measuring the composition of gases. The instrument on the
left is made of brass, the one on the right of glass. Cavendish's work on electricity, with the exception of two papers containing relatively unimportant matter, remained in the possession of his family until 1879, when the papers were edited by James Clerk Maxwell as the Electrical Researches of the Hon. Henry Cavendish. Cavendish investigated the capacity of condensers and constructed a series of condensers with which he measured the capacity of various pieces of apparatus using the "inch of electricity" as the unit of capacity. He discovered specific inductive capacity and measured this quantity; he showed that electric charges are confined to the surface of a conductor and that the inverse square law of force between charges holds to within 2%. He introduced the idea of potential under the name of "degree of electrification" in a paper published in 1771 under the title "Attempt to Explain Some of the Principal Phenomena of Electricity by Means of an Elastic Fluid." He also investigated the power of different substances to conduct electrostatic discharges (Philosophical Transactions, 1775) and completed an inquiry which amounted to an anticipation of Ohm's Law. Cavendish's last major achievement was his series of experiments to determine the force of gravitational attraction between two small bodies. (Philosophical Transactions, 1798). The apparatus he employed was devised by John Michell, but Cavendish had the most important parts reconstructed to his own designs. He suspended a light metal rod by a fine wire attached to its center. At each end of the rod, he fixed a small lead ball. He then brought two large lead balls close to the smaller ones but on opposite sides of the rod. The gravitational attraction between the large and small balls had the effect of turning the rod; by measuring the resulting twist in the wire, Cavendish was able to work out the strength of the gravitational force. With this information and Isaac Newton's equation of the law of gravitation, Cavendish showed that the mass of the earth was around 6.5 million billion tons and had a density about 5.5 times that of water. These figures are actually very close to the best estimates made by modern scientific methods. Other publications of his later years dealt with the height of an aurora seen in 1784 (Philosophical Transactions, 1790), the civil year of the Hindus (ibid, 1792), and an improved method of graduating astronomical instruments (ibid, 1809). Cavendish using his
specially-designed torsion beam apparatus to determine
the force of gravitational attraction between two small
bodies. Henry Cavendish died alone at Clapham Common, his home, on February 24, 1810. He was buried in the family vault at All Saints' Church, Derby. A Life, written by George Wilson and published for the Cavendish Society in 1851, contains an account of his writings, both published and unpublished, together with a critical inquiry into the claims of all the alleged discoverers of the composition of water. The remainder of Cavendish's papers was placed at the disposition of the Royal Society by the Duke of Devonshire. In 1921 the previously published work, together with a number of unpublished experiments, appeared under the title The Scientific Papers of the Honourable Henry Cavendish, F.R.S.; Vol. I, The Electrical Researches, revised with preface and notes by Sir J. Larmor; Vol. II, Chemical and Dynamical, edited by Sir Thomas Edward Thorpe, with additions by Charles Chree and others. Some of Cavendish's instruments are preserved in the Royal Institution, London, and his name is commemorated in the Cavendish Physical Laboratory at Cambridge University, which was built by the 7th Duke of Devonshire. SOURCE SEE ALSO |
| SKC Films Library
>> Science >> Chemistry >> Biography This page was last updated on 10/09/2017. |